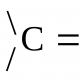Validation of the methods of calisthenic designation is carried out for. Mezha kіlkіsnogo vyznachennya. View of the bright houses
The skin instrumental method is characterized by the same level of noise, due to the specificity of the vimiruval process. To that, start the boundary of the zmistіv, lower for the yak speech vzagali can not be nadіyno revealed.
Intermediate manifestation Z min , P - the smallest value, for which, using this method, you can know the presence of a component from a given confidence level.
Mezha vyyavlennya mozhe buti tasks and minimal analytic signal y min , which can be vpevneno vіdіznyati vіd signal kontrolny dosvіdu - y background.
Statistical methods, from zastosuvannyam Chebishev's nervousness, it was brought to the conclusion that it is possible to distinguish between the manifestations, crusting with viraz
De s background is the standard definition of the analytical signal to the background; S-coefficient of sensitivity (sometimes called simply “sensitivity”), vin characterizes the response of an analytical signal to a component. Coefficient of sensitivity - the value of the first relative graduating function at a given designated concentration. For rectilinear graduation graphs - ce tangent kuta badly:


(respect: do not confuse sensitivity coefficientS zі standard allowancess!)
Іsnuyet and іnshі ways to rozrahunka mezhі vyyavlennya, ale tserіvnyannya vikoristovuyut most often.
In the case of a chemical chemical analysis, it is necessary to induce a range of appointments instead of concentrations. Він means the area of the value of the designated values (concentrations), transferred by this method and surrounded by the lower and upper boundaries of the designated concentrations.
Analysis h n or better m n component, which is assigned to this method. For the lower boundary of the appointed zmіstіv choose that minimum amount or concentration, as it can be calculated from an acceptable standard intake
 . .
. .
Butt
In retail, the mass concentration of saline was determined by the spectrophotometric method, vimiruyuchi optical width of the products, fermented as a result of the reaction of the interaction of the Fe 3+ ion with sulfosalicylic acid. For the purpose of graduating fallow, the optical width of the differences was increased due to the increasing (target) concentrations of the flood, enriched with sulfosalicylic acid.
The optical width of the difference (the control certificate for reactive, so without additional blinding, (background) was added 0.002; 0.000; 0.008; 0.006; 0.003.
Unravel between the revealed hall
Solution
1) As a result, calculate by the method of least squares (div. butt for control task No. 5) subtract the value for the graduation schedule.
Calculation of values for motivational graduation chart
2) Calculate the coefficient of sensitivity, that is the top coefficient of the grading fallow (S) according to the data in the table.

3) Calculable standard background signal rejection, what to become 0,0032 single optical thickness.
4) Between the manifestations of the time, mg / cm 3

Control room No. 6
Designate between the manifestations of the bay near the water.
Weekly data : the value of the optical width to the background (the difference in the difference) at the time of the graduation graph of the difference in the background was added 0.003; 0.001; 0.007; 0.005; 0.006; 0.003; 0.001; 0.005. The values of optical thickenings, depending on the concentrations of the sun in different areas are presented in the table of the control plant No. 5.
Calculate the difference between the manifestations of the gap in mg / cm 3 for the sensitivity coefficients S, calculated on the basis of data, taken away for the purpose of the schedule of graduation by the method of least squares at the end of control plant No. 5;
Between the kіlkіsny appointment
"...Межа кількісного визначення (LOQ) (в аналітичних визначеннях): найменша концентрація або аналізованої речовини в аналізованій пробі, яка може бути кількісно визначена з прийнятним рівнем точності та достовірності, що може бути продемонстровано за допомогою спільних випробувань лабораторій або іншого відповідного методу validation..."
Jerelo:
" PRODUCTS OF KHARCHOVI. METHODS OF ANALYSIS FOR THE PURPOSE OF GENETICALLY MODIFIED ORGANIZATIONS AND THE SUPPRESSION OF THEM PRODUCTS. HEALTH VIMOGI AND PURPOSE2(2)
(approved by Order of Rostekhreguluvannya dated December 25, 2008 N 708-st)
Official terminology. Akademik.ru. 2012 .
Marvel at the same "Mezha kolkisnogo vyznachennya" in other dictionaries:
boundary between the kіlkіsny appointment- 3.7 limit of quantification [LOQ] (limit of quantification): Ten times higher estimate of the standard sample mass yield. Note The LOQ value will change as a threshold value, if any weight is exceeded…
between repetitions- 3.7 recurrence intervals: The absolute difference between the results of the maximum and the minimum value from the designated number of viimiruvans, exceeding the recurrence points in terms of recurrence according to GOST R ISO 5725 1. Dzherelo ... Glossary of terms for normative and technical documentation
boundary between creativity- 2.9 between tests: The value, lower than the yak, with a change of 95%, the difference between the absolute values of the difference between the two results of testing, taking into account the minds of tests. Jerelo ... Glossary of terms for normative and technical documentation
between repetitions (zbіzhnostі)- 3.11 repeatability limit (repeatability limit): The value, as with a reliability limit of 95%, is not exceeded by the absolute value of the difference between the results of two vimiryuvan (or testing), we take away in the minds of repetition ... Glossary of terms for normative and technical documentation
Between internal laboratory precision- 3.11 Between the internal laboratory precision: Absolute difference between the two results of the analysis, taken away in the minds of the internal laboratory precision, which is allowed for the accepted accuracy R. Dzherelo ... Glossary of terms for normative and technical documentation
R- 2.19.2 performance margin R: The absolute value of the difference between the two test scores for intelligence performance (div. 2.19.1) with a confidence score of 95%. 2.19.1, 2.19.2 (Changed edition, title= Change No. 1, ІKS 12 2002). Glossary of terms for normative and technical documentation
МІ 2881-2004: Recommendation. DSI. Techniques for calciferous chemical analysis. Procedures for reviewing the acceptability of results in analysis- Terminology МІ 2881-2004: Recommendation. DSI. Techniques for calciferous chemical analysis. Procedures for reconsidering the acceptability of the results of the analysis: 3.17 critical difference: Acceptable for accepted acceptance is 95% absolute difference between… Glossary of terms for normative and technical documentation
GOST R 50779.11-2000 Statistical methods. Statistically keruvannya yakistyu. terms and definitions- Terminology GOST R 50779.11 2000 Statistical methods. Statistically keruvannya yakistyu. The terms and designation of the original document: 3.4.3 (upper and lower) boundary regulation Cordon on the control card, above the upper boundary, ... Glossary of terms for normative and technical documentation
GOST R 50779.10-2000 Statistical methods. Imovirnist and fundamentals of statistics. terms and definitions- Terminology GOST R 50779.10 2000 Statistical methods. Imovirnist and fundamentals of statistics. Terms and designation of the original document: 2.3. (general) sukupnіch Lack of all alone, which are looked at. Note For vertical dimension… Glossary of terms for normative and technical documentation
RMG 61-2003: Sovereign system for ensuring the unity of the world. Indicators of accuracy, correctness, precision of the methods of calculus chemical analysis. Assessment methods- Terminology RMG 612003: Sovereign system for the security of unity of war. Indicators of accuracy, correctness, precision of the methods of calculus chemical analysis. Evaluation method: 3.12 internal laboratory precision: Precision ... Glossary of terms for normative and technical documentation
MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION
ZAHALNA PHARMACOPEINA ARTICLE
Validation of analytical methods OFS.1.1.0012.15
Introduced first
Validation of the analytical methodology is the experimental proof that the methodology is suitable for the completion of tasks.
The main pharmacopoeial article regulates the characteristics of analytical methods, which are determined by the method of their validation, and the relevant criteria for the applicability of validated methods, which are recognized for the control of the quality of medicinal properties: pharmaceutical substances and medicinal preparations.
Validation is based on the methods of designing a house, including the methods of designing houses and the methods of designing intermediary. Authenticity methods are subject to validation if necessary to confirm their specificity.
When validating, an assessment of the analytical methodology is carried out according to the characteristics below, which are selected from the standard recommendations indicated in the table:
- specificity;
- detection limit;
- mezhі kіlkіsnogo vyznachennya (quantitation limit);
- analytical gallery (range);
- linearity (linearity);
- correctness (trueness);
- precision (precision);
- resistance (robustness).
Table 1 - Characteristics of methods that are determined during validation
|
name Characteristics |
Main types of methods | ||||
| Testing for accuracy | Side houses | kіlkіsne appointment | |||
| Kіlkіsnі methods | Mezha zmistu | The main fluent speech, normative components | Wild speeches at the test "Razchinennya" | ||
| Specification **) | So | So | So | So | So |
| Intermediate manifestation | Hi | Hi | So | Hi | Hi |
| Between the kіlkіsny appointment | Hi | So | Hi | Hi | Hi |
| Analytical area | Hi | So | Hi | So | So |
| Linearity | Hi | So | Hi | So | So |
| correctness | Hi | So | * | So | So |
| Precision :
- Repeatability (zbіzhnist) – promizhna (internal laboratory) precision |
|||||
| stamina | Hi | * | * | * | * |
*) can be appointed for the needs;
**) the lack of specificity of one analytical method can be compensated for by the variation of another analytical method.
Revalidation (re-validation) of methods is carried out when changing:
- technologies for maintaining the object of analysis;
- storage of medicines (object of analysis);
- earlier approved methods of analysis.
Specificity
Specificity - the purpose of the analytical technique to unambiguously assess the significance of speech in the presence of accompanying components.
The proof of the specificity of the methodology, which is valid, should be grounded on the basis of the analysis of the data on the analysis of model sums in the home warehouse.
The specificity of the validated methodology can also be brought to the surface by a statistical analysis of the results of the analysis of real objects, matching them to the variant and, in parallel, to the variant of the other, svіdomo specific methodology (methodology, specificity).
1.1 Testing methods for reference
The validated methodology (or the consistency of methods) is responsible for ensuring reliable information about the presence of the given speech in the substance or drug form for the presence in the warehouse of the components transferred by the recipe, which is for experimental confirmation.
The correctness of speech in the pharmaceutical substance or in the drug preparation is established in the same way with the standard sign or in the physical-chemical or chemical powers that are not characteristic of other components.
1.2 For methods of calisthenic designation and testing on houses
For the validated methodology of the calcissic prescription and the testing on the houses, however, it is necessary to evaluate the specificity of the chosen speech, so that it can be experimentally confirmed that the presence of the complementary components on the analyses does not affect the result.
It is allowed to assess the specificity of the validated methodology as a way to analyze the model sums in the warehouse, to avenge the speech, and to compare the results in the analysis of real objects, taking them away at once from the victories of the validated and specific methods. The results of various experiments may be statistically generalized.
The lack of specificity of testing may be compensated for by other (inshiny) additional testing.
With the validation of methods, as a matter of course, the signs of medicinal properties can be victorious, by means of accumulating houses in them, by the influx of extreme minds (light, temperature, moisture) or chemically modified in any other way.
For chromatographic techniques, show the difference between the two cavities, which are most closely related, at the same concentrations.
BETWEEN DESTINATIONS
Intervention - the value of the least amount (concentration) of the distinguished speech in the eye, as it can be revealed (or approximately estimated) from the standards of the validated methodology.
Between the manifestations in the variables assigned to the tables, it sounds like the concentration of the discriminated speech (in % of the speech cases per million - ppm).
Fallow according to the type of technique (visual and instrumental) vicorist different ways of delineating inter-phenomena.
2.1 For methods with a visual assessment of the result of the analysis
Carry out testing of samples with different viable quantities (concentrations) of speech, which are indicated, and set the minimum value, in which case the result of the analysis can be assessed visually. Tse є otsіnkoyu mezhі vyyavlennya.
2.2 For methods with instrumental evaluation of the result of analysis
2.2.1 According to the signal-to-noise ratio
Tsej pіdkhіd zastosovuєtsya to methodіv, kotrim poserіgaєtsya noise of the base line. Adjust the magnitude of the signals taken away for the control evidence for the recognition of low concentrations of speech, which are analyzed. Set the minimum amount (concentration) of the speech, which is pronounced, in the case, at a certain value of the ratio of the analytical signal to the level of the noise level 3.
The value of є estimating the inter-revealing is found.
2.2.2 For the value of the standard signal input and the cut coefficient of the calibration graph
Mezhu vyyavlennya (PZ) know for equal:
PZ = 3.3 S/b,
de S
b- Sensitivity coefficient, which is the ratio of the analytical signal to the value (tangent of the calibration curve).
Sі b
S S a a free member of this schedule. The difference between the value of inter-reflection, if necessary, can be confirmed by direct experiment with the amount (concentration) of speech, which are indicated, close to the known value of inter-reveal.
As a rule, as a rule, there is data about the applicability of the technique for the nadiy designation of speech in concentrations, which lie like a higher, so lower than the norm, it is better, the established specification, it is not necessary to determine the real difference between the manifestations for such a technique.
BETWEEN KILKIS DESIGNATION
The difference between the number of appointments is the least amount (concentration) of the speech in the eye, as it can be estimated from the standard of the technique, which is validated, with the necessary correctness and internal laboratory (industrial) precision.
The boundary of the calculus designation is the necessary validation characteristic of the methods that are distinguished for the assessment of small numbers (concentration) of speeches in the case of that, zocrema, for assessment in the place of houses.
Fallow, according to the type of vicorist method, there are such ways of distinguishing the inter-caliber appointment.
3.1 For methods of visual assessment of the result of the analysis
Carry out testing of samples with different volumes (concentrations) of speech, which are analyzed, and set the minimum value, in which case the result of the analysis can be subtracted visually with the necessary correctness and internal laboratory (industrial) prestige
3.2 For methods with instrumental evaluation of the result of analysis
3.2.1 According to the signal-to-noise ratio
Set the minimum concentration of the speech, which is indicated, in a clear way, when the value of the ratio of the analytical signal to the level of the noise becomes close to 10:1.
3.2.2 For the value of the standard signal input and the cut coefficient of the calibration graph
Between the calendar of appointments (EKO) to pay for the equal:
FSP = 10 S/b,
de S- Standard acceptance of the analytical signal;
b– coefficient of sensitivity, which determines the value of the analytical signal.
For the clearness of experimental data in a wide range of variable values Sі b can be evaluated by the path of the smallest squares.
For a linear calibration graph of the value S be equal to standard diet S a a free member of this schedule. Otrimane of the value of the inter-country variance, if necessary, can be confirmed by direct experiment with the quantities (concentrations) of speech, which are indicated, close to the known value of the inter-caliber quotation.
However, data on the adequacy of the technique should be used to analyze the speech in the concentration and lower than the one installed in the specification of the standard of yoga, to indicate the real value of the inter-caliber assignment for such a technique, as a rule, does not matter.
ANALYTICAL FIELD OF THE METHOD
The analytical area of the technique is the interval between the upper and lower values of the analytical characteristics of the measured component in the object of analysis (quantity, concentration, activity, etc.). At this interval, the results, the maintenance of different methods, which are validated, are the responsibility of the mother of the accepted level of correctness and internal laboratory (industrial) precision.
Up to the size of the analytical gallery of methods, the following are presented:
- Methods of caliber designation may be fixed in the interval of 80 to 120% of the nominal value of the analytical characteristic, which is to be determined;
- methods for evaluating the uniformity of dosing due to guilt and congestion in the interval from 70 to 130% of the nominal dose;
- Methods of calcu- lar typification, which are vicorated during the test "Designation", call the culprit but the stasis in the ranges of 50 to 120%, depending on the concentration of the deaf speech in the middle of the decency;
- methods of testing for purity due to faults in the intervals between "Between kіlkіsnogo vyznachennya" or "Bizhі vyyavlennya" up to 120% of the allowable house size, which is indicated.
The analytical area of the methodology can be set beyond the range of experimental data that satisfies the linear model.
Linearity
The linearity of the technique is the value of the manifestation of the linearity of the analytical signal in terms of concentration or the amount of speech, which is significant, in the analysis of samples in the boundaries of the analytical area of the technique.
When validating the methodology, the linearity in the analytical area is experimentally re-evaluated by the analysis of the analytical signals for at least 5 samples with different quantities, or the speech concentrations are determined. Experimental data are processed by the least squares method with the best linear model:
y = b · x + a,
X- how many or the concentration of speech, which is distinguished;
y- The value of vіdkuku;
b- Cut coefficient;
a- Volny member (OFS "Statistical analysis of the results of a chemical experiment").
Responsible buti foreclosed and submitted value b, a that correlation coefficient r. Most often, vicorists are lineal fallows, the average is 0.99, and less for the analysis of trace quantities, linear fallows are considered, which is 0.9.
In some cases, the possibility of a linear approximation of experimental data is only possible after their mathematical transformation (for example, logarithm).
For some methods of analysis, which are based on principles, they cannot be based on a linear fallacy between experimental data, the concentration of a quantity of speech is determined by using a variety of calibration non-linear graphs. With any graph of the analytical signal occurrence, depending on the quantity or concentration of the speech, which is indicated, there may be approximations by an appropriate non-linear function with the least squares method, which is shown for the obviousness of the program-free program.
CORRECTNESS
The correctness of the methodology is characterized by considering the average result of the appointment, vykonanyh s її vykoristannyam, the type of value that is taken for the purpose.
The validated technique is recognized as correct, as the values that are accepted as true, lie in the middle of the most reliable intervals in the average results of the analyses, taken experimentally for the given technique.
To assess the correctness of the methods of calculus appointment, the following approaches are used:
a) analysis of the widely validated methods of standard words or model sums with the volume (concentration) of speech that is distinguished;
b) equalization of the results, taking away from the alternatives of the validated methodology and the specific methodology, the correctness of which was established earlier;
c) a review of the results of the development of the linearity of the methodology, which is valid: if the largest term in the equation, induced in division 5, is not statistically significant in zero, then the comparison of such a methodology gives results, in the absence of a systematic pardon.
For approaches “a” and “b”, it is possible to represent data taken from the data as equal linear fallow (regression) between experimentally known and true values. For which equality, the hypotheses about the equality of unity to the tangent of the kuta are reconsidered b and about the equality of zero of a free member a. As a rule, if hypotheses are recognized as correct at the level of supremacy, which is 0.05, then the choice of the validated methodology gives the correct results, so that the results are correct.
PRECISION
The precision of the technique is characterized by the variability of the results, which vary according to the value of the average result. The world of such a rise is the value of the standard allowance for the result of a given award, taken away for the selection of a great obligation.
Precision is evaluated for any method of calculation for the results of no less than three values for the skin of three equal values (lower, middle and upper), which lie at the boundaries of the analytical region of the technique. Repeatability can also be assessed for whether or not the methodology of the calculus designation for the results of at least six designations for clear speech, which is considered to be close to nominal. In some cases, the assessment of precision can be carried out according to the results of the processing of experimental data by the least squares method, as specified in the GPM Statistical processing of the results of a chemical experiment.
Precision can be achieved on the same marks and can be evaluated in three options:
- Yak repetition (zbіzhnist);
- as an internal laboratory (industrial) precision;
- Yak interlaboratory precision.
The results of the assessment of the method of analysis for skin and of the options for precision sound are characterized by similar values of the value of the standard deviation to the result of the specified appointment.
Listen to the developments of the original methodology, the repetition (feasibility) of the results, which are awarded to the winners, is indicated. For the necessary inclusion of the expanded methodology to the normative documentation, the internal laboratory (industrial) precision is assessed additionally. Interlaboratory precision (relevance) of the methodology is assessed when it is submitted to the draft of the global pharmacopoeial statute, pharmacopoeial statute, or regulatory documentation for the standard pharmacopeia.
7.1 Repetitiveness
The repeatability of the analytical technique is evaluated by independent results, taken in the same regulated minds in the same laboratory (the same vet, the same owner, the same set of reagents) in the intervals of a short interval of an hour.
7.2 Internal laboratory (industrial) precision
Internal laboratory (industrial) precision of the methodology, which is validated, evaluated in the minds of the work of one laboratory (different days, different days, different days).
7.3 International laboratory precision
Interlaboratory precision (perfection) of the technique, which is validated, is evaluated during testing in different laboratories.
RESISTANCE
The stability of the validated methodology is the cost of saving the knowledge of the optimal (nominal) indicators, indicated by the tables, with possible small differences in the minds of these minds, the analysis is carried out.
The stability of the method is not the result of the very easy control of the minds of the analysis carried out. Tse rіzko soon will be needed for a special stamina.
Resilience is to be blamed for only being in quiet moods, if the technique being validated is based on victoria, especially sensitive to the best minds of methods of analysis, such as chromatography and functional analysis. For consumption, the assessment of the stability of the technique is carried out at the stage of development. It is possible that the stability of the methodology is not high, the re-verification of the appurtenance is obov'yazkovo without intermediary in the process of practical vikoristannya.
Rechecking the applicability of the analytical system
Re-verification of the applicability of the analytical system is the same re-verification of the main variables before it. The system, the applicability of which is being revised, is the collection of specific devices, reactives, standards and analysis. Vymogy to such a system should be concretized in the global pharmacopoeial statute on an appropriate analytical method. In this way, the re-verification of the applicability of the analytical system becomes a procedure that is included in the methodology, that is validated.
Submission of validation results
The protocol for the validation of the analytical method is guilty of revenge:
– її new description, sufficient for the implementation and reflection of all minds, necessary for analysis;
- Evaluation of characteristics;
– all the primary results that have advanced to the statistical processing of the data;
- the results of statistical analysis of data taken experimentally in the course of research or revision of the methodology that is validated;
– illustrative materials, such as copies of chromatograms, taken by high-performance native chromatography or gas chromatography; electrophoregrams, electronic and infrared spectra; photographs or small chromatograms taken by the methods of thin-ball or paper chromatography; little titration curves, calibration graphs;
- Visnovok about the applicability of the validated methodology for inclusion to the normative document.
Validation materials for other analytical methods should be formalized as a joint statement about validation.
COLLEGE
RISHENNYA
Vidpovly, to the Statt 30 Treaty on the єvrazi Ekononichi Union VID 29 Age 2014 Ta to paragraph 2 of Statti 3 Funds of the Rules of the Obiga Putting at the framework of the Ekonomichny Union 23 Akhodnya, 2014 Rocky
wrote:
1. Approve the Guidelines for the validation of analytical methods for testing drug tests.
2. Tse Decision is gaining rank in 6 months from the date of the first official publication.
Head of the Board
Eurasian Economic Commission
T.Sarkisyan
A guide to the validation of analytical methods for testing drug tests
APPROVED
Decisions of the Board
Eurasian Economic Commission
date 17 lipnya 2018 roku N 113
I. Gale positions
1. У цьому Посібнику визначаються правила валідації аналітичних методик проведення випробувань лікарських засобів, а також перелік характеристик, що підлягають оцінці під час валідації зазначених методик та включенню до реєстраційних досьє, що подаються до уповноважених органів держав - членів Євразійського економічного союзу (далі відповідно - держави -member, Union).
2. The method of validation of the analytical methodology for conducting drug testing and documenting confirmation of applicability for target recognition.
II. Appointment
3. For the purposes of this Helper, it is clear that they mean the following:
"аналітична методика" (аnalytical procedure) - методика проведення випробувань лікарських засобів, яка включає докладний опис послідовності дій, необхідних для виконання аналітичного випробування (у тому числі опис підготовки випробуваних зразків, стандартних зразків, реактивів, використання обладнання, побудови кривувальної кривої, використовуваних розрахункових formulas toscho);
"reproducibility" - power, which characterizes the precision in interlaboratory tests;
"range of stagnation (analytical area)" (range) - the interval between the largest and the smallest concentrations (quantity) is determined by speech at the time (including the number of concentrations), for which it is shown that the analytical technique has acceptable accuracy, accuracy;
"linearity" (linearity) - is directly proportional to the stagnation of the analytical signal in terms of concentration (amount) of the speech at the point in the range of the congestion (analytical area) of the technique;
"vіdkrittya" (recovery) - spacing between the average and true (reference) values with the correction of the avіdpovіdny avіrchihі intervals;
"repeatability (intra-assay precision)" - precision of the technique when vikonnnі repeated tests in the same working minds (for example, by one and the same analyst or a group of analysts, on the same possession, with one and the same reagents (toshcho) stretching a short interval for an hour;
"correctness" (accuracy, trueness) - proximity between the accepted true (reference) values and the rejected values, which are expressed by the value of the openness;
"between the number of appointments" (quantitation limit) - the smallest amount of speech in the eye, as it can be quantified with the highest precision and correctness;
"between manifestations" (detection limit) - the least amount of pronounced speech in the eye, as it can be revealed, but it is neobov'yazkovo exactly how much it is marked;
"precision" (precision) - the degree of closeness (level of expansion) of the results (value) between the series of vimiriv, carried out on anonymous samples, taken from one and the same sample, in the minds proponated by the method;
"проміжна (внутрішньолабораторна) прецизійність" (intermediate precision) - вплив варіацій усередині лабораторії (різні дні, різні аналітики, різне обладнання, різні серії (партії) реактивів тощо) на результати випробувань ідентичних зразків, відібраних з однієї і тієї ж серії;
"specificity" (specificity) - the ability of the analytical technique to unambiguously evaluate speech independently from other speeches (houses, degradation products, additional speeches, matrix (middle) and other), present in the tested sample;
"stіykіst (robustness)" (robustness) - the building of the analytical technique, but with the stability to the influx of small changes, which are asked in the minds of the testing, as if it indicates on її nadіinіst with the sizable (standard) vikoristannі.
III. Types of analytical methods for validation
4. Our Posibnik considers 4 widest types of analytical methods to be validated:
a) testing for identification (reference);
b) testing for designation of a small house (quantitative tests for impurities content);
c) limit testing for the control impurities;
d) a number of tests (for morning or activity) for the identification of the active part of the molecule of the intoxicating speech in the test sample.
5. All analytical methods, victorious control of the quality of medicinal products, must be validated. This Posibnik does not consider the validation of analytical methods for types of testing, which are not included up to paragraph 4 of this Posibnik (for example, testing for sizing or sizing the size of particles (dispersity) of pharmaceutical substances and in.).
6. As a rule, testing for identification (reference) is based on the same powers (for example, spectral characteristics, chromatographic behavior, chemical activity, etc.) of tested and standard samples.
7. Testing for the determination of a small house instead of a house and testing for the determination of a boundary house at the sample, directing to the correct description of the indications of the purity of the sample. Vymogi before validation of the methods of designation of the border designation of houses in the sample.
8. Techniques of kіlkіsnіkh vyprobіvаnі sprаvіvаnі іn vimіruvannіа instead of rhechovіnі, scho vynaєєєєє, vіprobіvanom zrazku. At the same Posibnik, according to the number of appointments, the number of main components of the pharmaceutical substance is understood. Similar validation parameters are determined according to the definition of fluent speech or other components of the medicinal preparation. It is allowed to test the parameters of the validation of the calculus designation in other analytical methods (for example, when testing for variance).
Appointment of analytical methods can be clearly defined, the oscills depending on the deposit of a variety of validation characteristics, if they can be assessed for the duration of the validation.
9. Evaluation of the following types of validation characteristics of the analytical method:
a) correctness (accuracy (trueness));
b) precision (precision):
repetition (repeatability);
intermediate (intermediate precision);
c) specificity;
d) detection limit;
e) the boundary of the quantity limit (quantitation limit);
f) linearity (linearity);
g) stagnation range (analytical area) (range).
10. The most important validation characteristics for the validation of various types of analytical methods are indicated in the table.
table. Validation characteristics for validation of different types of analytical methods
Validation | Type of analytical method |
||||
characteristic | testing on | testing at home | kіlkіsnі testing |
||
(Reference) | kіlkіsne | border guard | rozchinennya (tilki vimir), vmist (activity) |
||
correctness | |||||
Precision | |||||
repetition | |||||
industrial precision | |||||
Specifics** | |||||
Intermediate manifestation | |||||
Between the kіlkіsny appointment | |||||
Linearity | |||||
zasosuvannya range | |||||
________________
* As indicated by workmanship, it is not necessary to indicate industrial precision.
** The lack of specificity of one analytical method can be compensated for by the choice of one or more additional analytical methods.
*** You may need it in some situations (for example, if the boundary is revealed and the boundary between the houses is close together).
Note. "-" - the characteristic is not evaluated, "+" - the characteristic is evaluated.
Appointments perelіk slid to consider as a typical pіd hour of validation of analytical methods. It is possible to blame, which can be blamed on a person who has been primed with a vibrator of a medical care. Such a characteristic of the analytical technique, like stability (robustness), is not indicated in the table, but it should be looked at at the final stage of the development of the analytical technique.
Re-validation (revalidation) may be necessary in such situations (but do not combine them):
change of scheme for the synthesis of pharmaceutical substances;
change in the warehouse of the medicine;
change of analytical methods.
Re-validation will not be carried out, as the vibrator has given a good rounding. It is obligatory to re-validate according to the nature of the change.
IV. Methodology of validation of analytical methods
1. Key points to the methodology of validation of analytical methods
11. In which distribution, characteristics have been induced that are safe during the validation of analytical methods, and also induced recommendations for the establishment of different validation characteristics of the skin analytical method.
12. In some cases (for example, for the proof of specificity) for the safety of the quality of a pharmaceutical substance or a drug preparation, it is possible to use a number of analytical methods.
13. It is necessary to submit and analyze all the relevant data, the selection of the validation hour, and the formulas, revisions for the analysis of the validation characteristics.
14. It is allowed to win other entries, lower entries, contributions to your Helper. For the choice of the procedure and the protocol of validation, the applicant was liable. In this case, the main meta-validation of the analytical method is considered to confirm the applicability of the method for target recognition. Looking back at your ability to approach analytical methods for biological and biotechnological preparations, you can review them according to the descriptions of your Posibnik.
15. By the end of the last study of the validation characteristics, we have to test the standard values with the given characteristics, which is documented. The necessary purity level of standard objects should be deposited in accordance with the purpose of recognition.
16. Different validation characteristics can be seen in the next sections of this section. The structure of this division determines the process of development and evaluation of the analytical methodology.
17. The experimental work should be planned in such a way that the validation characteristics of the test can be performed immediately, taking into account the above data about the feasibility of the analytical method (for example, about specificity, linearity, range of accuracy, correctness and precision).
2. Specificity
18. It is necessary to check the specificity for the first time of validation and testing for identification, houses and kіlkіsne designation. Procedures for confirming the specificity of the deposit in the form of a target recognition of the analytical technique.
19. The method of confirming the specificity of the deposit in the field, for the completion of which the analytical method is recognized. It is not always possible to confirm that the analytical technique is specific to the given language (it is vibratory). And here it is recommended to win 2 and more analytical methods.
The lack of specificity of one analytical method can be compensated for by the choice of one or more additional analytical methods.
20. Specificity for different types of testing means onset:
a) when testing for identification - confirming that the technique allows you to identify the very speech that is being identified;
b) when testing on houses - confirmation of the fact that the technique allows you to correctly recognize houses at a glance (for example, testing on a half-day, important metal, instead of oversized retailers);
c) in case of small tests - confirmation of the fact that the technique allows you to restore the activity of the speech itself in the speech itself.
Identification
21. The task of testing for the identification of the mother's fault is the difference between structurally similar spores, which may be present in the sample. The vibrancy of the analytical technique can be confirmed by a way of eliminating positive results (possibly by a way of matching with a given standard sign) for signs, in order to avenge the assignments of the components, and negative results for signs, which do not avenge yoga.
22. To confirm the presence of chibnopositive results, testing for identification can be carried out for speeches with a close bud or speech, which support this speech.
23. The choice of speeches, which potentially zavazhayut the conduct of testing, is guilty of buti priming.
Kіlkіsne vyznachennya and testing on houses
24. When confirming the specificity for the analytical method, the chromatographic subdivision should be submitted with representative chromatograms with the appropriate significance of the individual components. It is necessary to win similar approaches to other methods based on rozpodil.
25. Critical problems in chromatography lead to marriage on a higher level. At the time of critical subdivisions, the value of the distribution of the 2 most closely related components was installed, but the value of the distributive property was set.
26. When choosing a non-specific method of kіlkisny designation, it is necessary to establish additional analytical methods and confirm the specificity of the whole complex of methods. For example, even though the release of a pharmaceutical substance is carried out by the titrimetric method, it can be supplemented with the same tests on the houses.
27. Pіdhіd аl similar to а kіlkіsny appointment, so try аt а house.
The presence of bright houses
28. For the obviousness of the signs of the houses, the specificity of the analytical technique is determined in the attack:
a) in case of a slurry appointment, it is necessary to confirm the vibrancy of the speech designation in the presence of houses and other components of the speech. Practically, it is necessary to add a way to the srazka (pharmaceutical substance or a medicinal preparation) houses and (or) additional speeches in a significant quantity and for the obviousness of proving the validity of their contribution to the result of a calculus speech;
b) when testing for houses, the specificity can be introduced by way of adding to the pharmaceutical substance, or the medicinal case of houses in the same number of cases, and for the obviousness of proving the subdivision of these houses one in one and (or) in the other components of the sample.
View of the bright houses
29. Yakshcho Standard Trucks Domita subgrades of Degradsii, the specificity of the blessing of Porivnyan, the result of the Vyprobuvan samples, the house of the subgrades, the results of the Inshovanny methodology (storage, pharmaco -pymacopea of the same In the case of different types of habits, it is standard to turn on the samples, which have recognized the savings in the singing stressful minds (light, heating, moisture, acid (basic) hydrolysis and oxidation).
30. At the time of the appointment, it is necessary to equalize 2 results.
31. At different tests on the houses, the profiles of the houses were sorted.
32. To prove the purity of the peak of the assigned speech, only one component should be additionally carried out for the purity of the peaks (for example, the whisker of diode-matrix detection, mass spectrometry).
3. Linearity
33. Linear fallow should be assessed within the limits of the range of analytical methods. Її it is possible to confirm without intermediary on the pharmaceutical substance (through the development of the main standard difference) and (or) on the four pieces of the piece (model) sums of the components of the medicinal preparation, the vicorist method has been proponated. The remaining aspect is allowed to vary depending on the range of the zastosuvannya (analytical gallery) of the technique.
34. Linearity is visually assessed according to the graph of the presence of an analytical signal as a function of the concentration or the quantity of the assigned speech. For the obviousness of a clear linear occurrence, the results should be processed using the most appropriate statistical methods (for example, the calculation of the regression line by the least squares method). To improve the linearity between the results of calculus assignment and sample concentrations before regression analysis, it may be necessary to mathematically reshape the results of testing. The results of the analysis of the regression line can be tested for the mathematical evaluation of the degree of linearity.
35. For the validity of the linearity of the data, the tests were carried out according to the mathematical revision before the regression analysis.
36. For PIDARENNYA LININOSTI BUTY VIKENSHENSIA TO COUFITICINT KORELATICIA ABOTITICINT DEMERMINATSIA, VILING member of the LINININENELE, Tangens Kuta Nakhilishkova Suma Sumlma, and Takozh, and Takozh, and Takozh, and Takozh.
37. Although the linearity is not expected for any kind of mathematical transformations (for example, when validating immunoanalytical methods), the analytical signal must be described using an additional function of the concentration (amount) of the designated component in the sample.
V. Stagnation range (analytical area)
39. The range of appraisal of the analytical technique to deposit in the form of recognition and depends on the twisted linearity. At the borders of the range of congestion, the technique is guilty of ensuring the need for linearity, correctness and precision.
40. As the minimum allowable faults, however, the following ranges of congestion (analytical areas) of analytical methods are considered:
a) for the calciferous designation of intoxicating speech in a pharmaceutical substance or in a medicinal preparation - in concentration (smist) 80 wdsotkiv to a concentration (smistu) 120 wdsotkiv in nominal concentration (smistu);
b) for homogeneity of dosing - in the concentration (change) 70 doses to the concentration (change) 130 doses, as for the medicinal product, a wider range of fallow in the medicinal form (for example, dosed inhalers) is not primed;
c) for testing for distribution - ± 20 cm (absolute) within the nominal range of testing. For example, as a result of the specifics of the drug with modifications of vilification, the range of 20 v/dsotkiv for the first year to 90 vdsotkiv of the declared amount for 24 years is to be blamed, the validation range of zastosuvannya is to be blamed for the range of 0 to 110 vdsotkiv of the declared amount;
d) for the designation of houses - the number of inter-development houses up to 120-hundreds value, assigned to the specification;
e) for houses, if they may be supernaturally strong, or they may have a toxic or non-permissive pharmacological effect, between the manifestations and the intercalation of the responsibility, but in proportion to that, on which the houses are to be controlled. In order to validate the methods of testing for houses, which will be completed at the hour of development, you may need to set an analytical area near the front (possible) boundary;
е) якщо кількісне визначення та чистота вивчаються одночасно за допомогою одного випробування і використовується лише 100-відсотковий стандарт, лінійна залежність має бути у всьому діапазоні застосування аналітичної методики починаючи з порога інформування для домішки (відповідно до правил вивчення домішок у лікарських засобах та встановлення вимог до them at the specifications, which are approved by the Eurasian Economic Commission) up to 120-hundredths of a sum, designated at the specification for the kіlkіsny appointment.
VI. correctness
41. Correctness can be established for the entire range of testing of the analytical technique.
1. Terms of designation of active pharmaceutical ingredients
Pharmaceutical substance
42. You can win a bunch of ways to assess correctness:
testing of the analytical method to the analyzed substance with the highest degree of purity (for example, to the standard material);
comparing the results of the analysis, deducting from the variations of the analytical methodology that is validated, and the results, deducting for the help of the methodology, the correctness of such a view, that (or) independent methodology.
Visnovok about correctness can be developed after the installation of precision, linearity and specificity.
Likarskiy zasib
43. Can you find some ways to evaluate correctness:
zastosuvannya analytical methods to piece (model) sums of components in the medicinal preparation, in the way it was added to the back of the line to see the amount of speech, which is indicated;
for the presence of signs of the essential components in the medicinal preparation, it is possible to add a delay in the quantity of the pharmaceutical substance to the medicinal preparation, or to match the results, to take away for the help of an additional technique, the correctness of such a technique independently (or)
Visnovok about the correctness can be developed after the designation of precision, linearity and specificity.
2. Kіlkіsne appointed house
44. Correctness is judged on samples (pharmaceutical substances and medicinal preparations), to which the number of houses is added.
45. Due to the presence of zrazkіv domіshok and (or) products of degradation, the results are acceptable and equal to the results, taken away for the help of an independent technique. It is allowed to use an analytical signal of speech, which is not.
46. It is necessary to indicate a specific way of expressing instead of individual houses or their sums (for example, in mass windows or in windows in terms of height to the peak area, but in all slopes of the main speech).
47. Correctness is evaluated not less than for 9 and 3 different concentrations, which cover the entire range of ingestion (then repeat 3 concentrations and 3 for skin concentration). The appointed responsibility includes all stages of the methodology.
48. The correctness is shown by the value of the score in the scores for the results of the number of words, added to the number of words to the analyzed section, or by the difference between taking the average and the true (reference) values from the improvement of the other words.
VII. Precision
49. Validation of testing on the kіlkіsne of designation and houses of transmission of designation of precision.
50. Precision is established on 3 levels: repetition, intermediate precision and repetition. The precision of the following is to be established for the help of homogeneous authentic sights. At the time of impossibility of trimming a uniform mark, it is allowed to designate precision for the help of piece-prepared (model) marks or the size of the mark. The precision of the analytical technique, as a rule, is expressed by the magnitude of the dispersion, the standard deviation, or the coefficient of variation of the series of experiments.
VIII. Repetitiveness
3
IX. Industrial (internal laboratory) precision
52. The steps of the established industrial precision lie in the minds of the victorious analytical methods. The Applicant is at fault for putting influencing factors on the precision of the analytical method. Typical relevant (variable) factors are different days, analytics, possession and other. Vivechati zaznachenі vplivi okremo is not necessary. With the introduction of various factors, it is necessary to win over the planning of the experiment.
X. Creativity
53. Performance characterizes precision in an interlaboratory experiment. Vіdtvoryuvanіst sіd vyznachit razі standardizatsії analytic methods (for example, when included to the Pharmacopoeia of the Union or the pharmacopoeia of the member states). The inclusion of data about the confirmation of the registration dossier is not included.
XI. tribute
54. For dermal type of precision, it is necessary to specify a standard respiration, a standard respiration (coefficient of variation) and a confidence interval.
XII. Intermediate manifestation
55. Possibly varying approaches to the designation of intervening deposits depending on whether the technique is instrumental or non-instrumental. It is allowed to vicariate on other occasions.
XIII. Visual assessment
56. Visual assessment can be scored for both non-instrumental and instrumental methods. Between the manifestations are established by the way of the analysis of samples with the known concentrations, the speech and the designation of the yogo minimal amount are indicated, in which case it is reliably indicated.
XIV. Evaluation of the inter-demonstration of the "signal/noise" method
57. Tsej pіdkhіd zastosovuєtsya less to analytical methods, for which the noise of the base line is suspected.
58. The determination of the "signal / noise" is carried out by the method of equalization of signals, taking samples from known low concentrations, taking signals from blank samples, and setting the minimum concentration, with which the speech can be reliably detected. To assess the inter-influence, the value of the signal-to-noise ratio in the range of 3:1 to 2:1 is taken into account.
XV. Evaluation of the inter-development behind the standard deviation of the analytical signal and the weak grading curve
59. The boundary between manifestations can be turned by the coming rank:
de:
60. The value of k is calculated from the grading curve for speech, which is displayed. Rating s can be changed in a number of ways:
b) according to the graduation curve. The next thing to analyze is the grading curve, prompted for understanding from the local speech, which is shown to be close to the inter-events. As a standard deviation, you can use a superfluous standard deviation of the regression straight line, or a standard deviation of the line point from the axis of the ordinates (standard deviation of the free member of the linear regression).
XVI. tribute
61. It is necessary to indicate between the manifestations and the method of yoga determination. As a rule, the difference between the manifestations is grounded on the visual assessment, or the "signal / noise" assessment, given by the relevant chromatograms, is considered sufficient for the assessment.
62. Even if the significance of the inter-expression is taken away by the path of rozrahunka or extrapolation, the assessment is due to buti confirmed for the help of an independent test of a sufficient number of signs from the most significant speech, which is unimportant to an insignificant inter-existence.
XVII. Between the kіlkіsny appointment
63. The boundary between the designation and the necessary validation characteristics of the methods that are used for the designation of a low volume of speech in the eye, the cream for the designation of houses and (or) products of degradation.
64. It is possible for a kіlka to go to the appointment of an inter-kіlkіsnogo appointment, depending on whether the method is instrumental or non-instrumental. It is allowed to vicariate on other occasions.
XVIII. Visual assessment
65. Visual assessment can be scored both for non-instrumental methods and for instrumental ones.
66. Between the caliber designation, it is necessary to establish a path for the analysis of samples with the given concentrations, the speech and the assessment of the minimum amount are determined, in which case the speech is determined according to the calculus designation with the accepted correctness and precision.
ХІХ. Evaluation of the inter-caliber designation of the "signal / noise"
67. Tsej pіdkhіd zastosovuєtsya less to the methods of vimіryuvan, in which one is afraid of the noise of the base line.
68. Vynovannaya “signal/noise” is carried out by the method of porvynnia of the Wimyryuvani, Odrimani VID Zdimimi with the low concentrations of Vishni Richovini, with the ivermances of the UDD of the UNITRITE, the MINIMAL CONTRICTIONAL PRISENISED, under the YAKILY VITOSHIC. Zvichayne ratio "signal / noise" warehouse 10:1.
XX. Evaluation of the inter-caliber difference between the standard deviation of the signal and the weak grading curve
69. The border of the kіlkіsny appointment (PKO) can be expressed in such a rank:
de:
s – standard deviation of the analytical signal;
k is the tangent of the kuta of the grading curve.
70. The value of k is calculated from the grading curve for speech, which is displayed. Rating s can be changed in a number of ways:
a) for a standard blank sample. The value of the analytical signal is measured for a sufficient number of unfriendly samples, and the standard allowance for their value is provided;
b) according to the graduation curve. The next thing to analyze is the grading curve, prompted for understanding from the local speech, which is shown to be close to the middle caliber. As a standard deviation, you can use a superfluous standard deviation of the regression straight line, or a standard deviation of the line point from the axis of the ordinates (standard deviation of the free member of the linear regression).
XXI. tribute
71. It is necessary to indicate between the number of appointments and the method of determination.
72. It is necessary to confirm the difference between the number of milestones by the year for additional analysis of a sufficient number of samples from the chosen speech, which is closer to the middle of the country's designation, or close to a new value.
73. You can be friendly and other people, who wake up in the sights.
XXII. Stability (robustness)
74. Vivchennya stability (robustness) is necessary to be studied at the stage of development, it is obligatory to deposit according to the analyzed analytical method. It is necessary to show the superiority of the analysis for the smallest variations in the parameters (minds) of the technique.
75. As a result of vimiryuvan to lie in the minds of changes in the minds of the analysis of analytical methods, it is necessary to strictly control such minds, or to educate yourself later, come in at the hour of the testing.
76. With the help of the method of ensuring the validity of the analytical method in case of victoriousness, one of the most recent developments in stability (robustness) may be the establishment of a series of parameters in the applicability of the system (for example, testing for permission (resolution test)).
77. Big variations of parameters є:
stability of rozchinіv, yakі vikoristovuyutsya in analytical methods;
hour of extraction.
Variation parameters for native chromatography are:
changing the pH of the ruhom phase;
change in the warehouse of the roaming phase;
different columns (different series and posts);
temperature;
swidk_st rohomoї phase (shvidk_st flow).
Variation parameters for gas chromatography є:
different columns (different series and posts);
temperature;
gas-carrying speed.
XXIII. Assessment of system applicability
78. Evaluation of the applicability of the system to an invisible part of the wealth of analytical methods. The tests are based on the concept that the possession of electronic technology, analytical operations and analysis of the mind to establish a complete system and make the evaluation as such. Criteria for applicability of the system are to be established for a specific technique and to be deposited according to the type of analytical technique that is validated. Additional information can be taken from the Pharmacopoeia of the Union or from the pharmacopoeias of the Member States.
Electronic text document
preparations AT "Kodeks" and zvіreny for:
official website
Eurasian Economic Union
www.eaeunion.org, 20.07.2018